Whether it’s waking up with a migraine or experiencing rapid-onset pain, patients want relief—fast.1,2

Fast1
Patients may begin to feel migraine pain relief in as little as 10 minutes (17% of patients vs. 5% for placebo).*

Powerful1
~50% of patients experienced migraine pain relief 30 minutes after administration (47% vs. 15% for placebo).*

Simple
A convenient, ready-to-use auto-injector with:
- A concealed 29 G needle1
- A discreet, compact design3
- No refrigeration needed†
60% of patients experienced migraine pain relief 2 hours after administration (vs. 21% for placebo).1 Results from a single-attack, parallel group design study, where six different doses of sumatriptan injection, including 3 mg, were compared (n=30 in each group) with placebo (n=62).
*Time to onset and degree of pain relief varies by patient. †Store between 68°F and 77°F. Excursions permitted between 59°F and 86°F.

Zembrace® SymTouch® Offers the Lowest Dose in a Sumatriptan Auto-injector1
Minutes Matter
Proportion of patients with migraine pain relief by time with a 3 mg dose of sumatriptan injection (n=30).1 ‡
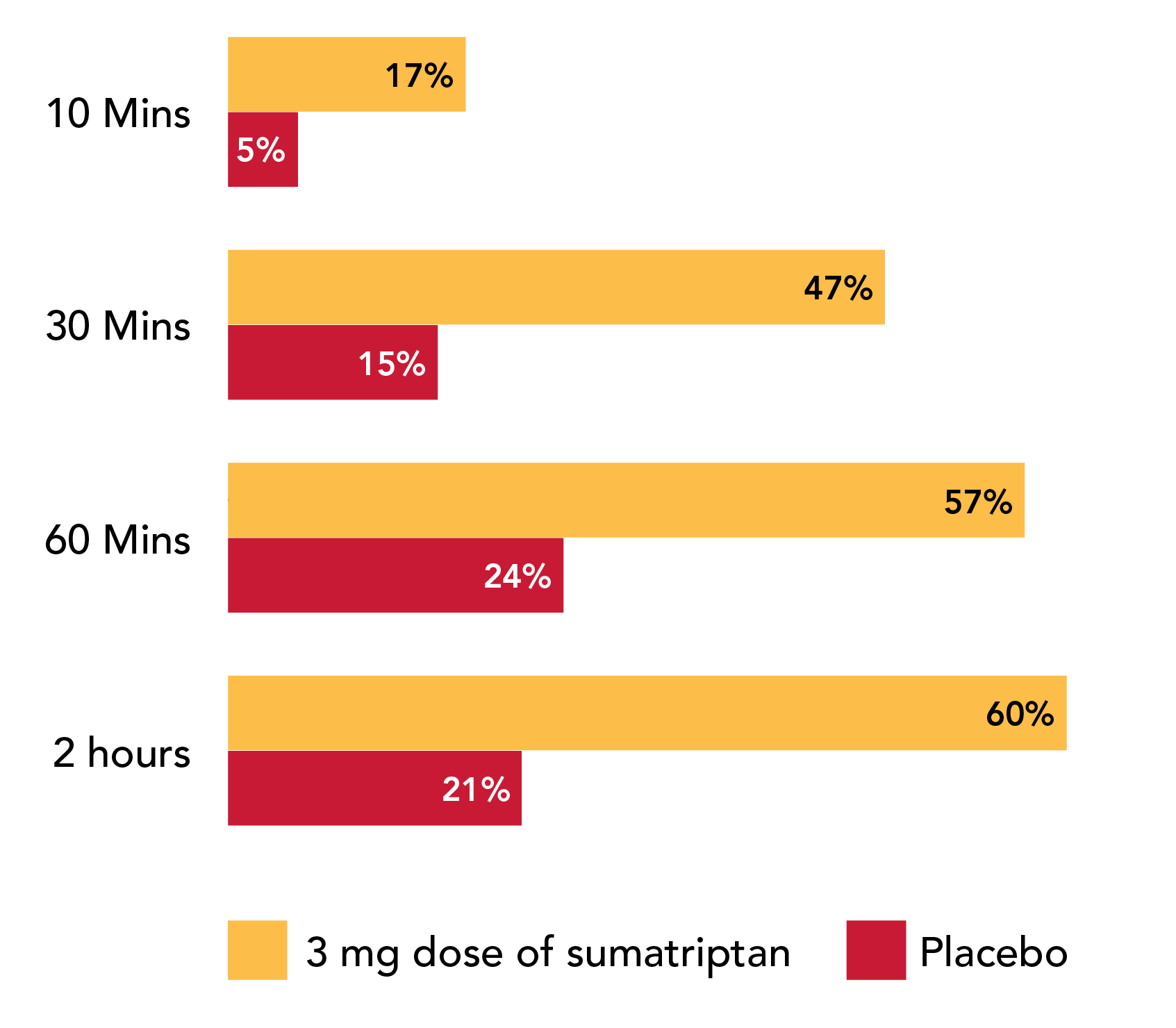
‡Results from a single-attack, parallel group design study, where six different doses of sumatriptan injection, including 3 mg, were compared (n=30 in each group) with placebo (n=62).
Use only if a clear diagnosis of migraine has been established. If a patient has no response to the first migraine treated, reconsider the diagnosis before Zembrace SymTouch is administered to treat subsequent attacks. Zembrace SymTouch is not indicated for the prevention of migraine attacks, and is contraindicated in patients with a history of hemiplegic or basilar migraines.
Clinical Evidence – RESTOR Study
Clinical support for the use of Zembrace SymTouch was provided by the RESTOR study and the open-label extension thereof.4,5 The RESTOR study was a multicenter, randomized, double-blind, placebo-controlled trial that evaluated the effectiveness and safety of Zembrace SymTouch for acute migraine treatment.4 A total of 268 participants with episodic migraine were randomized to receive either Zembrace SymTouch or a placebo for a single moderate-to-severe migraine attack.4
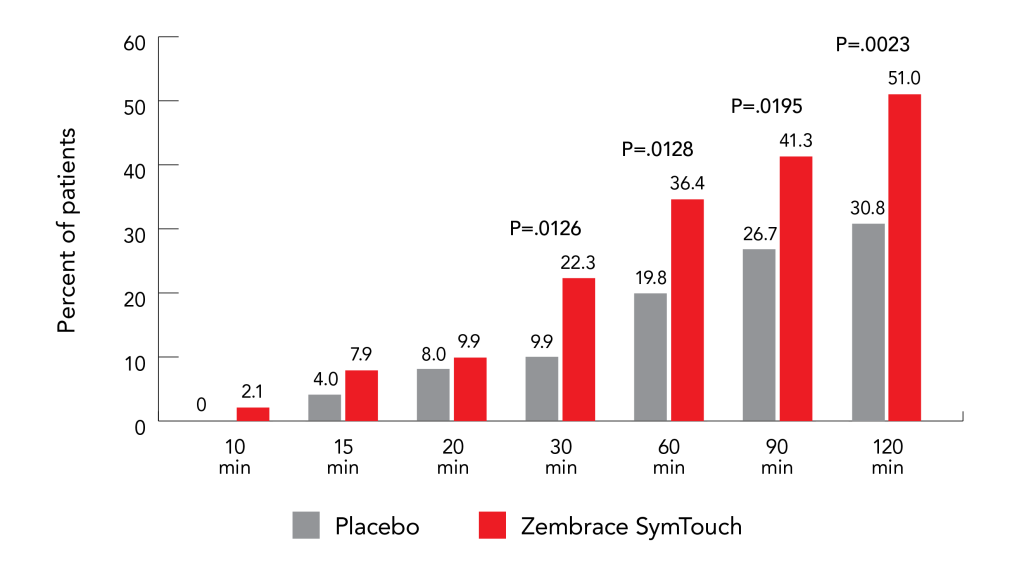
Results demonstrated that 51.0% of participants in the Zembrace SymTouch group achieved pain freedom at 2 hours compared to 30.8% in the placebo group (P = 0.0023). Zembrace SymTouch also showed significant superiority over placebo at earlier timepoints (as brief as 30 minutes) for pain freedom, pain relief, and MBS freedom.4
The RESTOR study concluded that Zembrace SymTouch is an effective and well-tolerated option for acute migraine management.4 Treatment-emergent adverse events (TEAEs) were reported by 33.3% of participants receiving Zembrace SymTouch compared to 13.4% in the placebo group, with the most common being injection site swelling (7.2%) and pain (7.2%). Importantly, triptan-related sensations such as chest discomfort were minimal in the Zembrace SymTouch group (0.9%).4
Clinical Evidence – RESTOR Open-Label Extension Study
The subsequent 8-week open-label extension study evaluated the efficacy, tolerability, and safety of Zembrace SymTouch in treating multiple migraine attacks.5 The study included 234 subjects with episodic migraine who treated a total of 848 migraine episodes with 1042 doses of Zembrace SymTouch.5 At 2 hours post-dose across four attacks, pain freedom rates ranged from 57.6% to 66.3%, and pain relief rates were between 81.7% and 88.4%.5 Freedom from the MBS, nausea, photophobia, and phonophobia also showed consistent efficacy across attacks. TEAEs were reported by 40.6% of subjects, with injection site reactions being the most common. Most TEAEs were mild, and only 2.1% of subjects discontinued due to adverse events.5 The study concluded that Zembrace SymTouch was effective, tolerable, and safe for acute treatment of multiple migraine attacks over 8 weeks, with consistent responses on pain and associated symptoms.
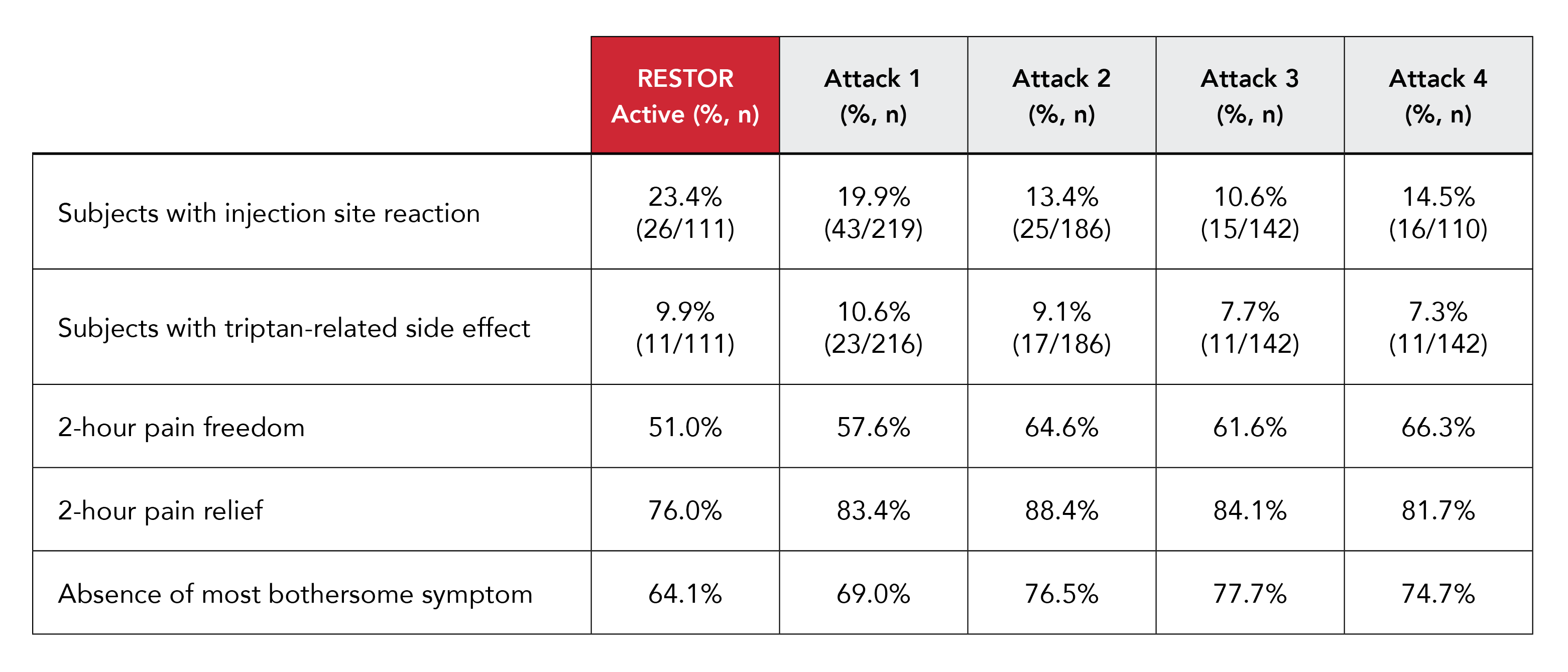
Together these findings suggest that the 3 mg dose of subcutaneous sumatriptan in Zembrace SymTouch offers a safe and effective alternative to higher doses for managing migraines.
Enhanced Tolerability with Zembrace SymTouch
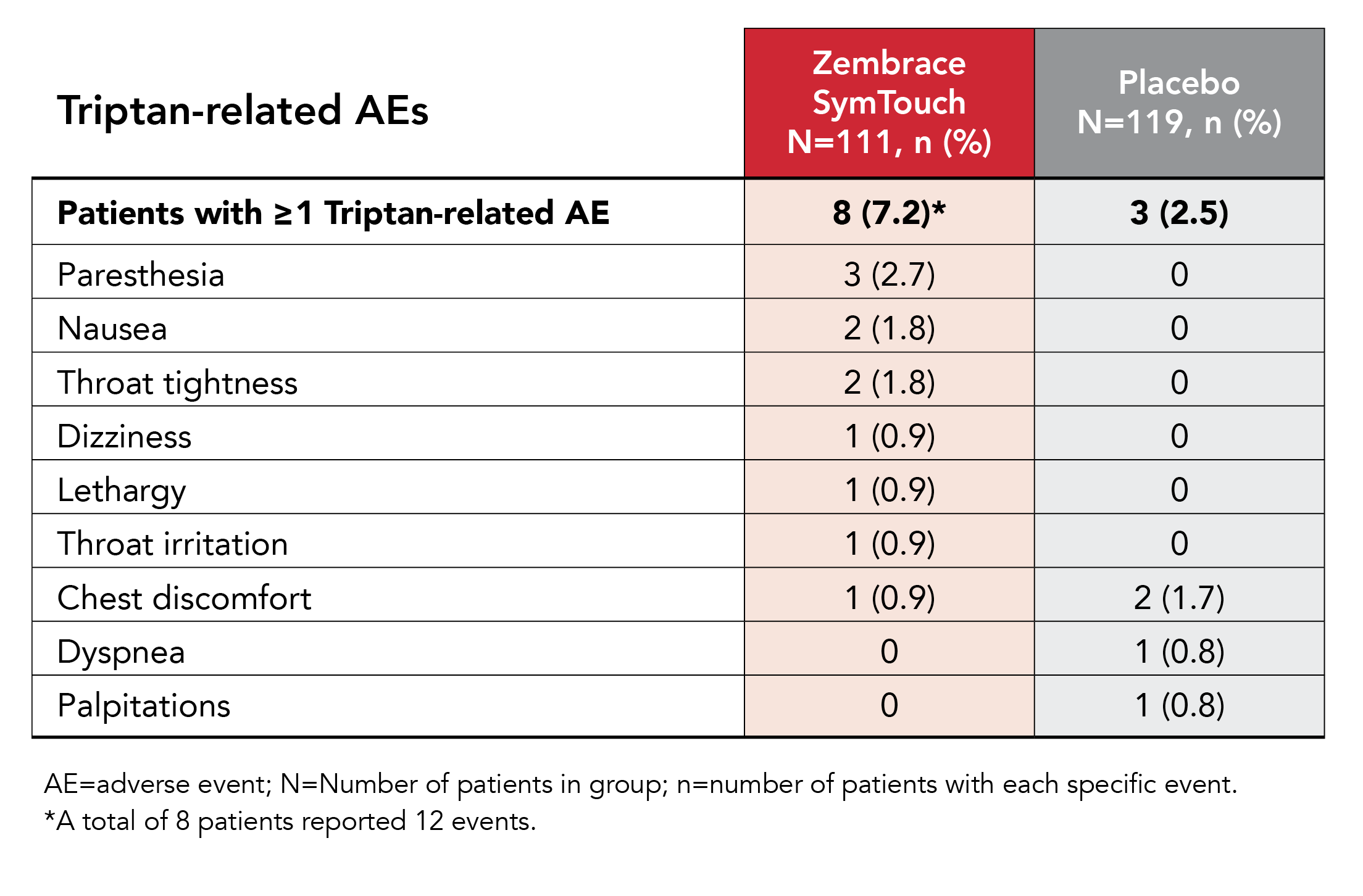
More than 90% of patients reported no triptan-related adverse events (AEs)
A low incidence of triptan-related AE has been observed in clinical trials4,5
- 2% of patients treated with Zembrace SymTouch reported ≥1 triptan-related AE versus 2.5% of patients who received placebo4
- No triptan-related AE exceeded an incidence of 3%4
- The incidence of triptan-related AEs decreased over multiple treated migraine attacks5
Multicenter, randomized, double-blind, placebo-controlled study that evaluated the safety and efficacy of Zembrace SymTouch compared with placebo in adults with episodic migraine with or without aura (N=230),4 followed by an 8-week open-label extension.5
Convenient To Carry and Easy-To-Use
Simple, two-step injection process with the smallest needle in a sumatriptan pen (auto-injector)1
Zembrace SymTouch Is RANKED FIRST6
Zembrace SymTouch was ranked first in performance and preference compared with other subcutaneous sumatriptan autoinjectors for all subjective factors, including:
